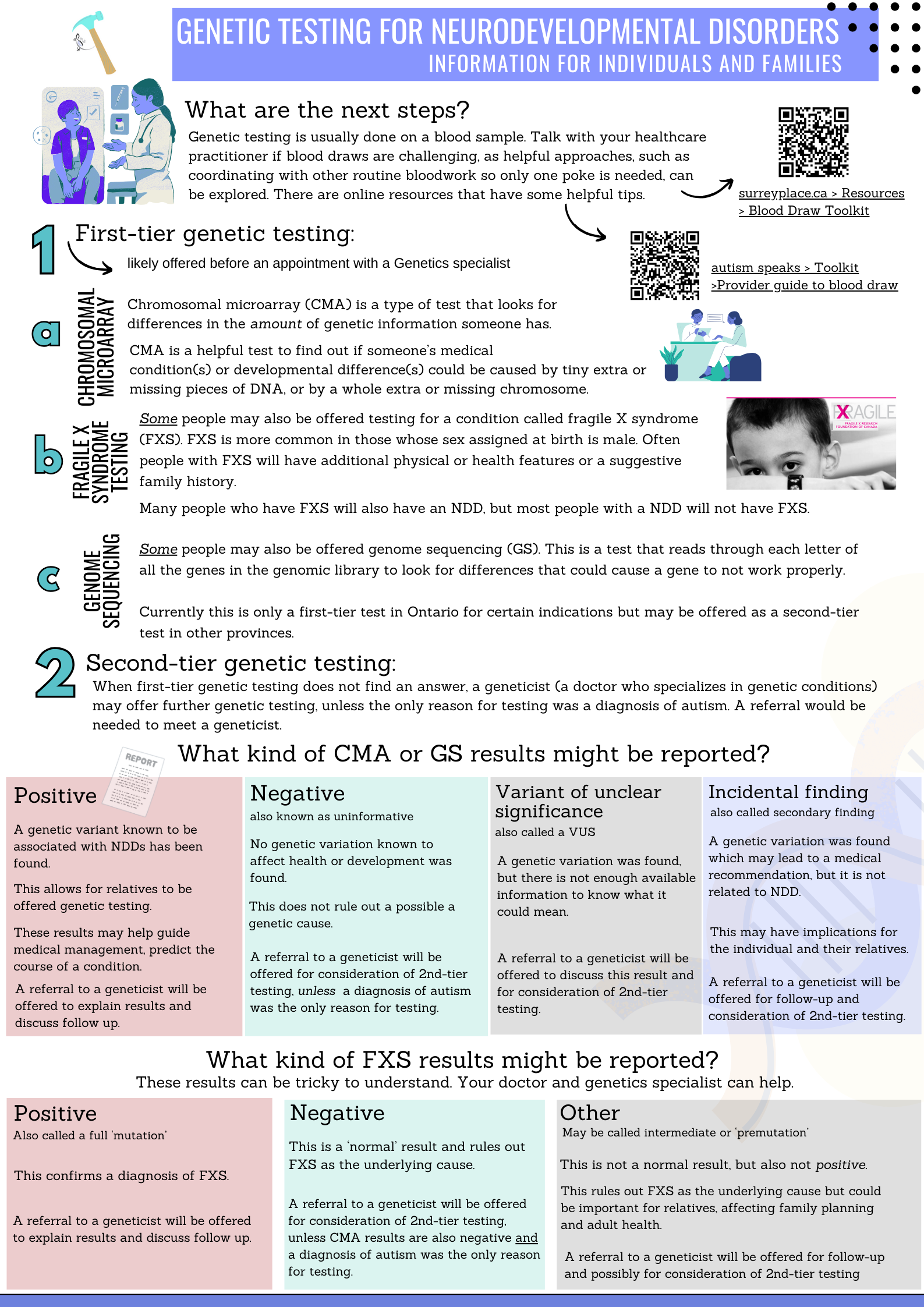
GECKO on the run: A 4-page, evidence-based summary for healthcare practitioners. Features a bottom line, red flags to consider genomic consultation or testing, surveillance and management recommendations. (June 2025)
News and Events
- Written by Super User
- Written by Super User
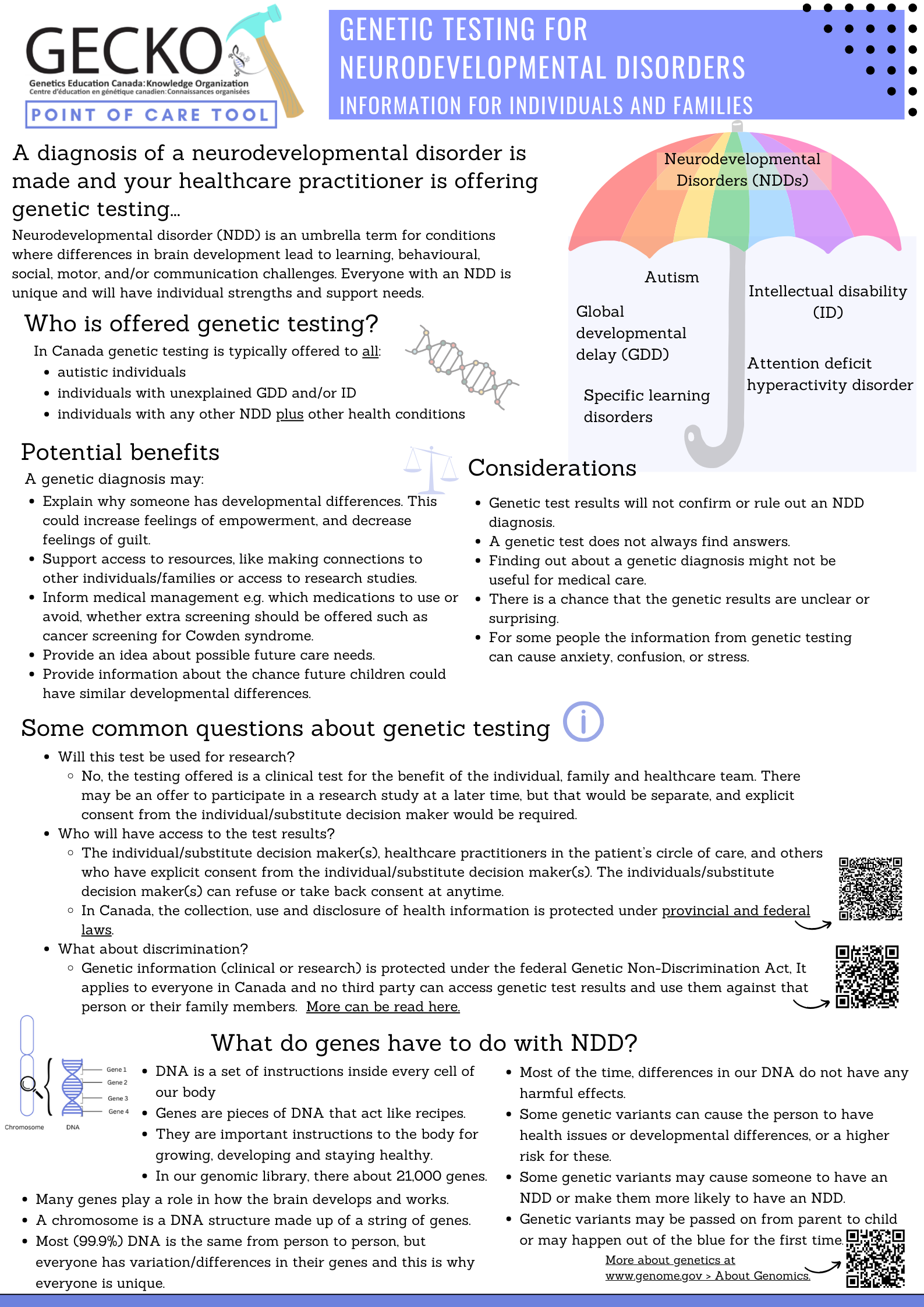
Point of care tool: Contains when to offer genetic testing, when to consider genetic testing and when genetic testing is not indicated. (June 2025)
- Written by Super User
Factor V Leiden
Point of care tool: Contains when to offer genetic testing, when to consider genetic testing and when genetic testing is not indicated. (June 2025)
GECKO on the run: A 4-page, evidence-based summary for healthcare practitioners. Features a bottom line, red flags to consider genomic consultation or testing, surveillance and management recommendations. (June 2025)
- Written by Super User
New study: An email-based, EHR-integrated strategy significantly improved family history documentation in primary care (16% vs 0.2%). Patients reported the approach was helpful and led to more screening and referrals. Seamless integration supports better preventive care. #Genetics #PrimaryCare #DigitalHealth #Genomics #FamilyHistory #FamilyHistoryIsTheFirstGeneticTest
Carroll et al Ann Fam Med 2025;23:399-406.
https://www.annfammed.org/content/annalsfm/23/5/399.full.pdf

- Written by Super User
Download and save the resource here (2025)
Developed with input from the Patient and Family Advisory Committee at the Children's Hospital of Eastern Ontario.
Download and save the resource here (2025)
Developed with input from the Patient and Family Advisory Committee at the Children's Hospital of Eastern Ontario.
- Written by morrison
Have some questions about how Canada’s genetic non-discrimination act works and what it means for your practice? Check out Canadian Family Physician for a free access article:
Cowan JS, Kagedan BL, Graham GE, Heim-Myers B, Bombard Y. Health care implications of the Genetic Non-Discrimination Act: Protection for Canadians' genetic information. Can Fam Physician. 2022 Sep;68(9):643-646. PMID: 36100377
- Written by morrison
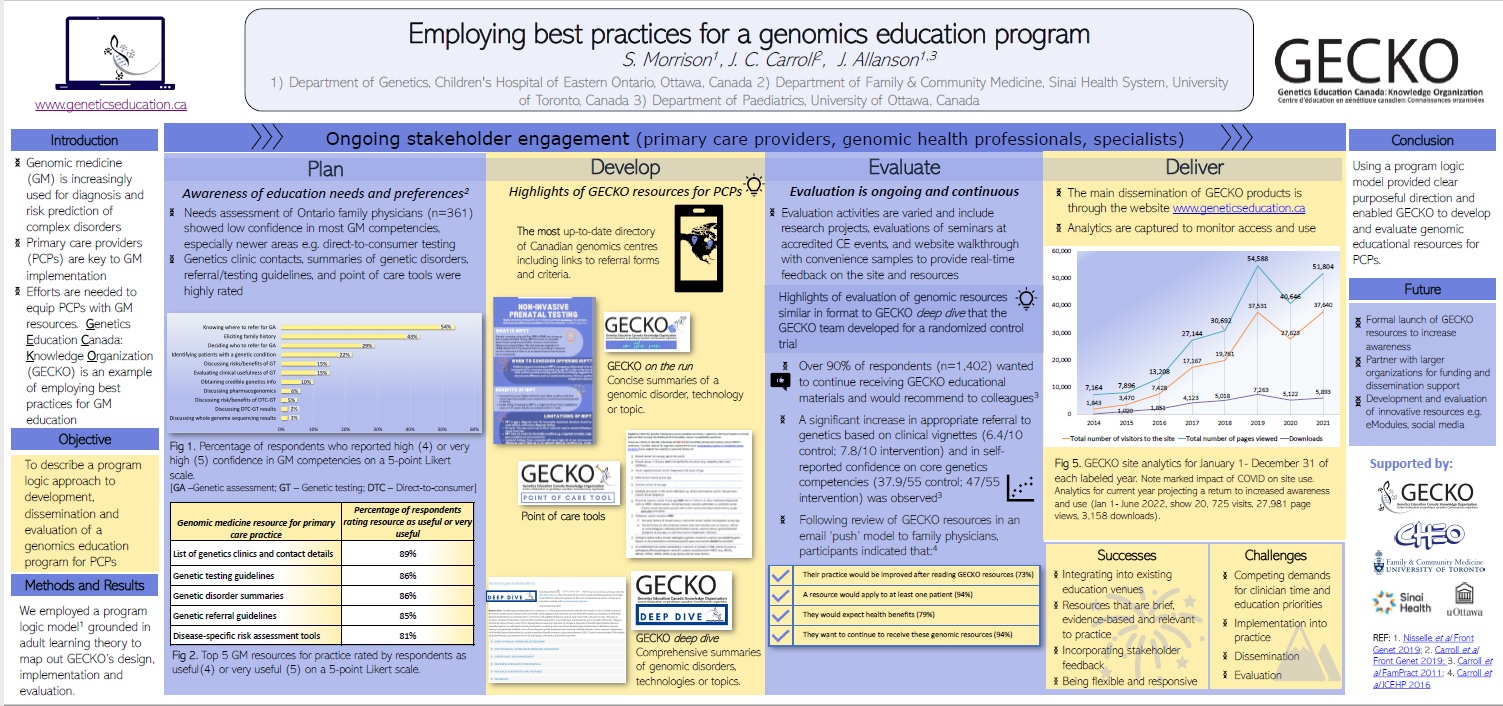
Ever wondered how we do what we do and do it so well? Check out our poster that highlights best practices for a successful genomics education program
- Written by morrison
Want to know what’s happening with the Canadian Familial Hypercholesterolemia Registry?
Check out the 2022 Annual Progress Report describing various initiatives, publications, highlighting the CardioRisk Calculator App which can be used to make a clinical diagnosis of FH for your patients.
![]()
- Written by Super User