Download the updated PDF here [2023]
Check out our new patient resource on NIPT (June 2024).
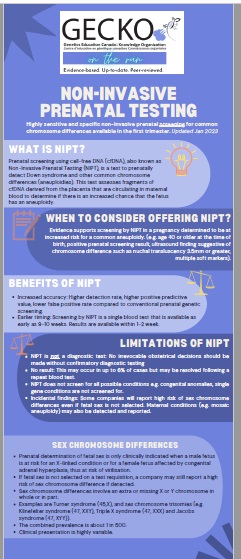
Highly sensitive and specific non-invasive prenatal screening for common chromosome differences, available in the first trimester. Updated Jan 2023.
A more comprehensive overview of NIPT can be found here in the GECKO deep dive.
Scroll to the bottom for links to your provincial prenatal screening programs and criteria for publically funded NIPT.
What is NIPT?
Prenatal screening using cell-free DNA (cfDNA), also known as Non-Invasive Prenatal Testing (NIPT), is a test to prenatally detect Down syndrome and other common chromosome differences (aneuploidies, i.e. trisomy 18). This test assesses fragments of cfDNA derived from the placenta that are circulating in maternal blood to determine if there is an increased chance that the fetus has an aneuploidy.
When to consider offering NIPT?
Evidence supports screening by NIPT in a pregnancy determined to be at increased risk for a common aneuploidy (e.g. age 40 or older at the time of birth, positive prenatal screening result, ultrasound finding suggestive of chromosome difference, such as nuchal translucency 3.5mm or greater, multiple soft markers). NIPT is funded in some provinces for those who meet high risk criteria. Others may choose self-pay for NIPT.
Benefits of NIPT
- Increased accuracy: Higher detection rate, higher positive predictive value, lower false positive rate compared to conventional prenatal genetic screening.
- Earlier timing: NIPT is a single blood test that is carried out as early as 9-10 weeks. Results are available within 1-2 weeks.
Limitations of NIPT
- NIPT is not a diagnostic test. No irrevocable obstetrical decisions should be made without confirmatory diagnostic testing.
- Uninterpretable result: This may occur in up to 6% but may be resolved following a repeat blood test.
- NIPT does not screen for all possible conditions. Congenital anomalies, single gene conditions are not screened for.
- Incidental findings: Some companies will report high risk of sex chromosome difference even if fetal sex identification is not selected. Maternal conditions (e.g. mosaic aneuploidy) may also be detected and reported.
Sex chromosome differences
- Sex chromosome differences involve an extra or missing X or Y chromosome in whole or in part.
- Examples are Turner syndrome (45,X), and sex chromosome trisomies (e.g. Klinefelter syndrome (47, XXY), Triple X syndrome (47, XXX) and Jacobs syndrome (47, XYY)).
- The combined prevalence is about 1 in 500.
- Clinical presentation is highly variable.
- Prenatal determination of fetal sex is only clinically indicated when a male fetus is at risk for an X-linked condition (e.g. Fragile X Syndrome) or a female fetus is at risk of congenital adrenal hyperplasia causing virilisation.
- If fetal sex identification is not selected on the test requisition, a company may still report a high risk of sex chromosome difference when detected.
Results
Negative/Low risk
- This is reassuring.
- Offer first trimester ultrasound (11 to 14 weeks gestation) for accurate dating, determination of twin chorionicity, early anatomy assessment and NT measurement.
- Offer second trimester ultrasound (18 to 22 weeks gestation) for evaluation of structural anomalies.
- Consider referral for genetics and/or maternal fetal medicine consultation depending on the reason for NIPT.
- e.g. for an ultrasound finding of an NT ≥ 3.5mm and negative NIPT, a detailed ultrasound, fetal echocardiogram and additional genetic investigations would be offered
Positive/High risk
- No irrevocable obstetrical decisions should be made in pregnancies with abnormal NIPT results without confirmatory diagnostic testing (CVS or amniocentesis) as false positive results do occur. The positive predictive value (PPV) is not 100% for any condition in any population.
- Offer genetic counselling and confirmation by diagnostic testing.
No result/uninterpretable
- This may occur in up to about 6% of cases. The laboratory will usually request a second blood sample, repeating the test at no extra cost. In 50-60% of cases a result will then be available.
- Most often test failure is due to low fetal fraction (amount of fetal cfDNA relative to pregnant person’s) which is influenced by factors such as early gestational age, high BMI, IVF or fetal aneuploidy. Other possible reasons are blood collection and/or transportation issues, technical issues e.g. a poor sample or quality assessment failure.
- When contemplating repeating the blood test, consider whether a delay in results/no result and further delay to diagnostic testing is important for time-sensitive decision making.
- If the NIPT result is not interpretable following second attempt, test failure may be due to low fetal fraction associated with an increased aneuploidy risk (~5%). Consider genetic counselling where diagnostic testing and ultrasound may be offered.
Additional screening by NIPT
- Currently no national or international organizations support routine clinical implementation of screening for additional conditions by NIPT.
- Examples of these conditions are microdeletion and microduplication syndromes, rare trisomies (e.g. trisomy 15 or 16), and structural anomalies to identify unbalanced chromosome rearrangements.
- The clinical utility and sensitivity have not been demonstrated.
Microdeletions and microduplications
- Microdeletion and microduplication syndromes are rare genetic conditions, occurring in 1 in 5,000 to 1 in 50,000 pregnancies, caused by very tiny extra or missing pieces of a chromosome.
- The imbalance of genetic information may have developmental and health implications.
- The most common example is 22q11.2 deletion or DiGeorge syndrome.
Private pay NIPT
- When public health care funding is not available, paying out of pocket may be an option.
- Cost and access vary by company, test selection and geographic location.
- GECKO does not endorse any particular company.
- Consider talking to your local prenatal genetic counsellor about what is available near you. See www.geneticseducation.ca > Clinics
Twins and multiples
- NIPT as a first trimester screening in twin pregnancies is appropriate and can complement first trimester ultrasound evaluation.
- Testing for sex chromosome differences is generally not available.
- High order multiples: Observed detection rates for trisomy 21 and other common aneuploidies are not available. Test failure rate is expected to be higher as the fetal fraction per fetus is lower.
In vitro fertilization
- NIPT for IVF pregnancies is the preferred method of prenatal screening.
- Diagnostic testing is not recommended unless NIPT screening result is high risk/positive, or ultrasound identifies anomalies consistent with a high risk of aneuploidy.
Below are links to provincial prenatal screening program and information about whether or not NIPT can be obtained with provincial funding: [Reviewed May 2024]
|
Province |
Provincial screening |
NIPT funded |
|
Alberta |
MyHealth Alberta Prenatal Screening https://myhealth.alberta.ca/genetics/prenatal-screening-and-testing |
No |
|
British Columbia |
Perinatal Services BC Prenatal Screening http://www.perinatalservicesbc.ca/our-services/screening-programs/prenatal-genetic-screening |
Yes, in some circumstances |
|
Saskatchewan |
Information for public |
No |
|
Manitoba |
Prenatal Genetic Screening & Diagnosis Service - Winnipeg Regional Health Authority (wrha.mb.ca) https://wrha.mb.ca/genetics-and-metabolism/prenatal-genetic-screening/ |
Yes, following positive maternal serum screen |
|
Maritimes (New Brunswick [NB], Nova Scotia [NS], Prince Edward Island [PEI]) |
IWK Prenatal Screening |
Yes, in some circumstances NB, discuss with maternal fetal medicine specialist NS and PEI criteria found here: IWK Health Centre - Clinical Genomics - NIPT Information for Care Providers (nshealth.ca) https://iwkhealth.ca/clinical-genomics/testing-menu/nipt-information-care-providers |
|
Newfoundland and Labrador |
contact local genetics centre |
Yes, criteria found here |
|
Ontario |
Prenatal Screening Ontario |
Yes, criteria found here |
|
Quebec |
Québec prenatal screening program |
Genetic services and test access and criteria for the Territories are generally administered through provincial programs
- Alberta and Northwest Territories and Nunavut (Kitimeot)
- British Colombia and Yukon
- Manitoba and Nunavut (Kiviliq)
- Ontario and Nunavut (Baffin)