Check out our new patient resource on NIPT (June 2024).
Download the updated PDF here [2022] or download the PDF en francais here [2015]. Prenatal screening using cell-free DNA (cfDNA), also known as Non-Invasive Prenatal Testing (NIPT), is a test to prenatally detect Down syndrome and other common chromosome differences. This test assesses fragments of cfDNA derived from the placenta that are circulating in maternal blood to determine if there is an increased chance that the fetus has an aneuploidy. NIPT should be considered in pregnancies at increased risk of aneuploidy (e.g. positive conventional prenatal screen [such as enhanced First Trimester Screening (eFTS), Integrated Prenatal Screening (IPS)/ Maternal Serum Screening (MSS)], or an associated ultrasound finding). NIPT has higher sensitivity and specificity for trisomy 21 (Down syndrome) and trisomy 18 than conventional screening tests, however it is not diagnostic. Positive results should be confirmed by diagnostic testing (chorionic villus sampling or amniocentesis) prior to any irrevocable action. Negative results are reassuring, significantly reducing the likelihood of common chromosome differences. Additional follow-up testing and consultation may still be indicated as NIPT does not screen for congenital anomalies or many other genetic conditions. Some provinces fund NIPT as a second-tier screen for persons who meet certain high-risk criteria. Those who do not meet criteria can pay for NIPT themselves. Price varies by company and test selection.
Scroll to the bottom for links to your provincial prenatal screening programs and criteria for publically funded NIPT.
What is Non-Invasive Prenatal Testing by cell-free DNA?
Non-Invasive Prenatal Testing (NIPT) by cell-free DNA (cfDNA) is a highly sensitive and specific way to screen for aneuploidies (an extra or missing chromosome)) in pregnancy. Common chromosome aneuploidies are trisomy 21 (Down syndrome), trisomy 18 (Edward syndrome), trisomy 13 (Patau syndrome). NIPT is a screening test providing a risk assessment, it is not a diagnostic test. NIPT is performed on a pregnant person’s blood sample and poses no harm to the pregnancy.
A meta-analysis reviewing clinical validation and implementation studies found that, in singleton pregnancies, the detection rates (DR) and false positive rates (FPR) were:1
◦ DR: 99.7% and FPR: 0.04% for trisomy 21
◦ DR: 97.9% and FPR: 0.04% for trisomy 18
◦ DR: 99.0% and FPR: 0.04% for trisomy 13
NIPT is not a diagnostic test. A positive/high risk NIPT result should be confirmed by diagnostic testing (chorionic villus sampling [CVS] or amniocentesis) prior to any irrevocable action.2
NIPT can also be used for sex chromosome identification for the purpose of fetal sex determination. This can be clinically important in pregnancies with an increased risk for an X-linked disorder (e.g. haemophilia A, Duchenne muscular dystrophy).
How does NIPT work?
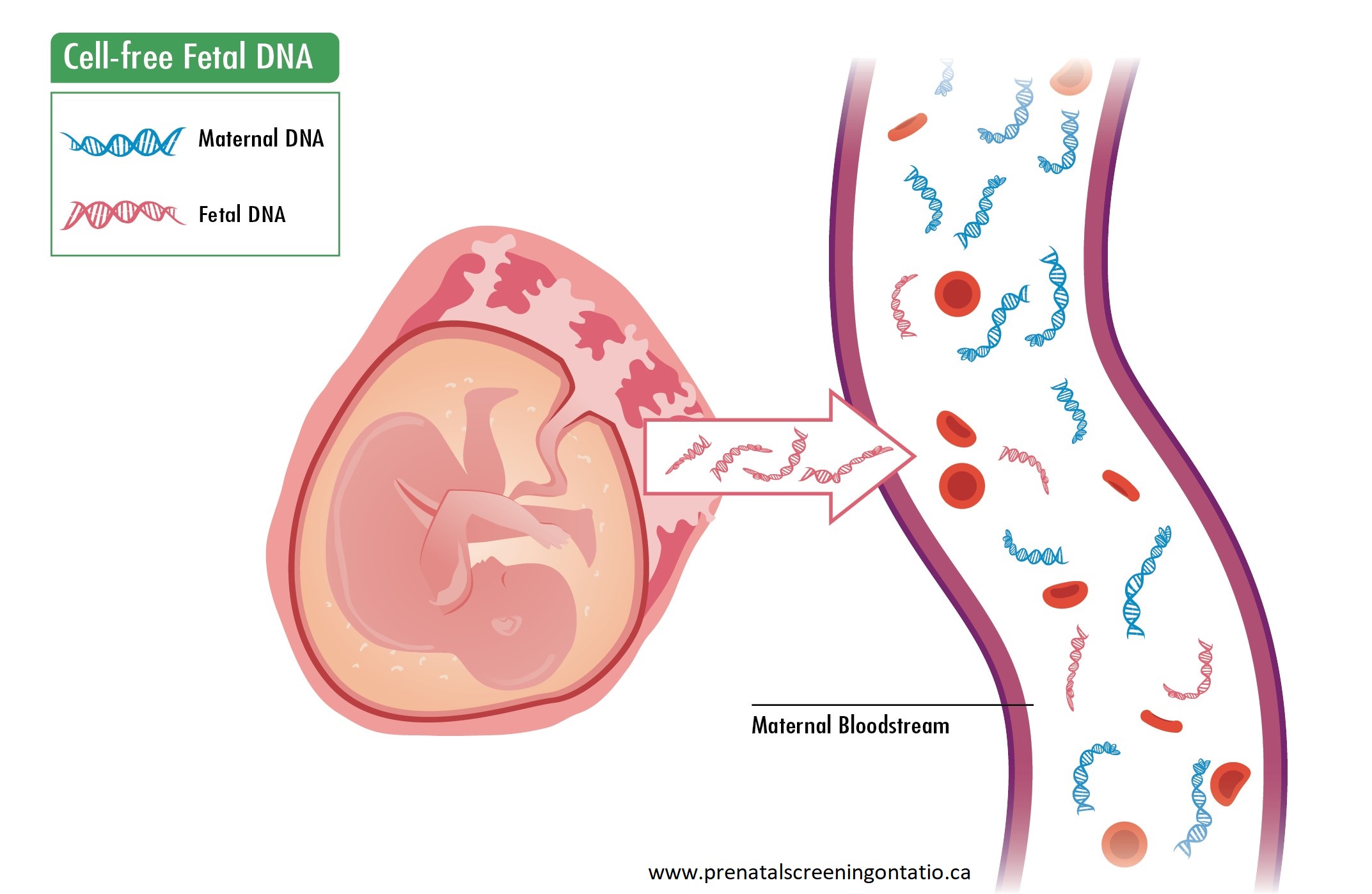 cfDNA refers to very small fragments of DNA not contained in a cell. They are always present in the blood stream. During pregnancy, some circulating cfDNA is derived from the placenta, and this is expected to represent the fetal genomic profile.
cfDNA refers to very small fragments of DNA not contained in a cell. They are always present in the blood stream. During pregnancy, some circulating cfDNA is derived from the placenta, and this is expected to represent the fetal genomic profile.
A blood sample is obtained from the pregnant person and the circulating cfDNA is analysed to assess the likelihood of extra or missing genomic information, as compared to the expected amount. Testing can be done as early as 9-10 weeks gestation (company specific) up to birth.
cfDNA from the pregnancy comprises only a small percentage (<10%) of the total cfDNA in a pregnant person’s blood. The quantity increases with gestational age. Various technologies and proprietary algorithms for analysis are used by each laboratory.1
A dating ultrasound is recommended prior to drawing the blood sample to: (1) ensure pregnancy viability, (2) obtain an accurate gestational age, and (3) exclude multiple pregnancies.
What are the Canadian recommendations on prenatal screening?
Current Canadian clinical practice guidelines by the Society of Obstetricians and Gynaecologists of Canada (SOGC) and the Canadian College of Medical Geneticists (CCMG) state that all pregnant persons in Canada, regardless of age, should be offered, through an informed counselling process, the option of a prenatal screening test for the most common fetal aneuploidies.2
All pregnant persons should be offered the options of:
1. No aneuploidy screening
2. Standard prenatal screening based on locally available programs e.g. enhanced First Trimester Screening (eFTS), Integrated Prenatal Screening (IPS)
3. Diagnostic testing (chorionic villus sampling (CVS) or amniocentesis) when appropriate indications are present e.g. a fetal nuchal translucency (NT) measurement of 3.5mm or greater or a congenital anomaly
4. NIPT screening where available, with the understanding that it may only be provincially funded when certain criteria are met
Additionally, where available, pregnant persons should be offered a first trimester ultrasound (at 11 to 14 weeks gestation) for accurate dating, determination of twin chorionicity, early anatomy assessment and NT measurement. A second trimester ultrasound (18 to 22 weeks gestation) should be offered for evaluation of structural anomalies. These ultrasounds should be performed at centres with expertise in fetal ultrasound.2
Note: The primary screening test for the detection of fetal structural abnormalities including open/closed neural tube defects (O/CNTDs) is the second trimester ultrasound. The primary use of maternal serum alpha fetoprotein for O/CNTD screening should be discontinued unless the pregnant person has a pre-pregnant BMI ≥ 35 kg/m2 or where access to timely and good quality ultrasound is limited.3
Red flags: When to consider offering NIPT and/or genetic consultation
SOGC and CCMG guidelines2 recommend all pregnant persons be offered the option of a prenatal screening test for the most common fetal aneuploidies (trisomies 21, 18 and 13). While offering NIPT as a primary screening method is not funded in most provinces, NIPT is available in many provinces as a second-tier screen. See the online resource for links to provincial prenatal screening programs and NIPT funding criteria.
Evidence supports screening by NIPT in a pregnancy determined to be at increased risk for a common aneuploidy.
In general, a pregnancy is considered to have an increased chance of fetal aneuploidy when:
 The pregnant person is 40 years of age or older at the time of estimated date of birth
The pregnant person is 40 years of age or older at the time of estimated date of birth  There is an abnormal serum screen result i.e. eFTS/IPS/MSS
There is an abnormal serum screen result i.e. eFTS/IPS/MSS The fetal nuchal translucency (NT) measurement is 3.5mm or greater
The fetal nuchal translucency (NT) measurement is 3.5mm or greater There is a history of a previous pregnancy or child with trisomy 21, 18 or 13
There is a history of a previous pregnancy or child with trisomy 21, 18 or 13 There are fetal congenital anomalies on ultrasound highly suggestive of trisomy 21, 18 or 13
There are fetal congenital anomalies on ultrasound highly suggestive of trisomy 21, 18 or 13  There are multiple soft markers2,4 on ultrasound which are highly suggestive of a specific aneuploidy
There are multiple soft markers2,4 on ultrasound which are highly suggestive of a specific aneuploidy There is a risk of carrying a male fetus with an X-linked condition (NIPT screening could be used for sex determination)
There is a risk of carrying a male fetus with an X-linked condition (NIPT screening could be used for sex determination)
As each prenatal genetics centre has its own referral criteria, abnormalities seen on ultrasound (e.g. congenital anomalies, NT ≥ 3.5mm or multiple soft markers) should be discussed with your local genetics centre or maternal fetal medicine specialist to decide whether: (1) a referral is appropriate, (2) NIPT screening could be offered first, (3) additional testing could be considered e.g. microarray, additional imaging.
Private pay NIPT
NIPT may be obtained by paying out-of-pocket when public health care funding is not available. Cost and access vary by company, test selection and geographic location. A recent Canadian study found that between January 1, 2016, and December 31, 2017, 23,845 pregnant persons in Ontario had NIPT screening and, of those, one third (7,931) were self-paid.5 GEC-KO does not endorse any particular company.
Before ordering NIPT for your patient, consider first talking to your local prenatal genetic counsellor about what is available near you. Genetics clinics across Canada often have a genetic counsellor available to answer questions from community providers and are happy to do so.6 Genetic counsellors may also be available for questions through your provincial prenatal screening program. See program links in Red flags: When to consider offering NIPT and/or genetic consultation.
When ordering NIPT as a private pay option you may wish to discuss with a company representative the considerations below.
What does the test result mean?
Depending on the company, results may be worded as: positive/negative; aneuploidy detected/no aneuploidy detected/aneuploidy suspected/borderline value; or high risk/low risk.

If the result is negative/low risk, this is reassuring.
Your patient should still be offered:
 • First trimester ultrasound (at 11 to 14 weeks gestation) and nuchal translucency measurement where available
• First trimester ultrasound (at 11 to 14 weeks gestation) and nuchal translucency measurement where available
• Second trimester ultrasound (18 to 22 weeks gestation)
• Depending on the reason NIPT was ordered, a referral for genetic and/or maternal fetal medicine consultation for discussion of additional testing and/or follow up
e.g. for an ultrasound finding of an NT ≥ 3.5mm, a detailed ultrasound, fetal echocardiogram and additional genetic investigations would be offered
 If the result is positive/high risk, follow-up genetic counselling is indicated and confirmation by diagnostic testing should be offered.
If the result is positive/high risk, follow-up genetic counselling is indicated and confirmation by diagnostic testing should be offered.
The SOGC/CCMG recommendations state that no irrevocable obstetrical decisions should be made in pregnancies with abnormal NIPT results without confirmatory invasive testing2 (CVS or amniocentesis) as false positive results do occur.
 While the detection rate of NIPT is quite high for most conditions, the positive predictive value (PPV) will vary based on the prevalence of (how common) the condition in a given population.
While the detection rate of NIPT is quite high for most conditions, the positive predictive value (PPV) will vary based on the prevalence of (how common) the condition in a given population.
The PPV is not 100% in any population.
Consider the PPV of the result for your patient for the condition screened positive.
When NIPT is positive for trisomy 21, the PPV is expected to be higher if the a priori risk (expected prevalence) is higher (e.g. 40 years or older at the time of estimated date of birth, a previous positive serum screen, a suggestive finding on ultrasound)
When the NIPT is positive for a much rarer condition such as trisomy 13 or a microdeletion (see Additional screening by NIPT) the PPV is expected to be lower.
 If there is no result, the laboratory will usually request that a second blood sample is drawn and the test repeated at no extra cost. Often (in 50-60%) a result will be available after a repeat blood draw.2
If there is no result, the laboratory will usually request that a second blood sample is drawn and the test repeated at no extra cost. Often (in 50-60%) a result will be available after a repeat blood draw.2
A request for repeat blood draw may occur in up to about 6% of cases, depending on the test methodology.1 A recent study looking at Ontario’s provincial data reported a 2.2% failure rate with no result after multiple blood draws.7
 Possible reasons for test failure are (1) blood collection and/or transportation issues, (2) technical issues e.g. a poor sample or quality assessment failure, and, most often, (3) low fetal fraction (amount of fetal cfDNA relative to pregnant person’s) which is influenced by a number of factors such as early gestational age, high BMI, or fetal aneuploidy.8-10
Possible reasons for test failure are (1) blood collection and/or transportation issues, (2) technical issues e.g. a poor sample or quality assessment failure, and, most often, (3) low fetal fraction (amount of fetal cfDNA relative to pregnant person’s) which is influenced by a number of factors such as early gestational age, high BMI, or fetal aneuploidy.8-10
 When contemplating repeating the blood test, the patient and provider should consider whether a delay in results/possibility of no results, and possible further delay to diagnostic testing is important to time sensitive decision making. If there is a priori low risk of aneuploidy and/or the pregnancy is at an early gestational age (e.g. 10 weeks), a delay in results may be acceptable. If the a priori risk of aneuploidy is increased, depending on the gestational age, moving ahead with diagnostic testing may be preferable for the patient.
When contemplating repeating the blood test, the patient and provider should consider whether a delay in results/possibility of no results, and possible further delay to diagnostic testing is important to time sensitive decision making. If there is a priori low risk of aneuploidy and/or the pregnancy is at an early gestational age (e.g. 10 weeks), a delay in results may be acceptable. If the a priori risk of aneuploidy is increased, depending on the gestational age, moving ahead with diagnostic testing may be preferable for the patient.
 If the NIPT result is not interpretable following a second attempt, test failure due to low fetal fraction is associated with an increased aneuploidy risk (~5%).2 Consider genetic counselling where diagnostic testing and ultrasound may be offered.
If the NIPT result is not interpretable following a second attempt, test failure due to low fetal fraction is associated with an increased aneuploidy risk (~5%).2 Consider genetic counselling where diagnostic testing and ultrasound may be offered.
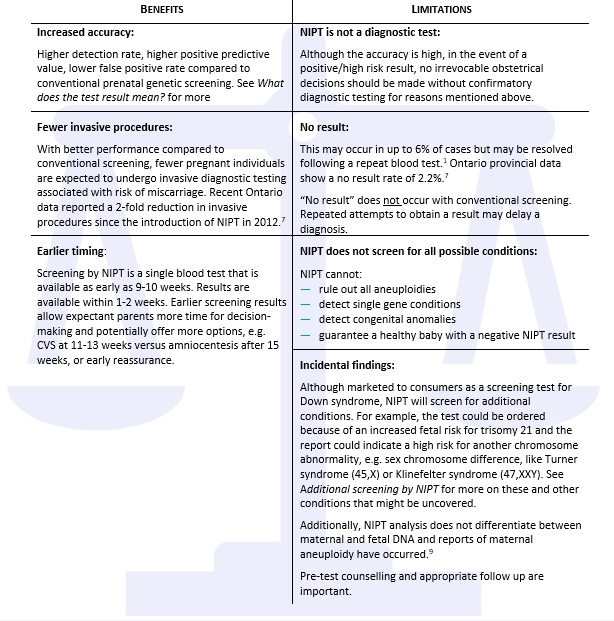
What are the benefits and limitations of NIPT?
What about NIPT screening for sex chromosome differences?
NIPT for detection of sex chromosome differences (SCD) is also possible and offered by all companies. Even if fetal sex identification is not selected on a test requisition, some companies may still report a high risk of sex chromosome difference when detected.
SCD involve an extra or missing X or Y chromosome in whole or in part. Examples of SCD are Turner syndrome (45,X), and sex chromosome trisomies (e.g. Klinefelter syndrome (47, XXY), Triple X syndrome (47, XXX) and Jacobs syndrome (47, XYY)). The combined prevalence is about 1 in 500. Clinical presentation is highly variable among and within conditions with SCD. Counselling is difficult and complex. While Turner syndrome may be better described,11,12 much of the literature to date reporting the clinical presentation of sex chromosome trisomies is flawed due to small numbers and ascertainment bias depicting the most severe presentations. Most individuals with sex chromosome trisomies (75-90%) are not diagnosed in childhood but may present in adulthood because of fertility problems or other issues,13-17 while the diagnosis of Turner syndrome is at a median age of 15 years11.
SOGC and CCMG state that prenatal determination of fetal sex is not clinically indicated, even when considering patient autonomy, except for assessing risk of an X-linked condition in a male fetus, or virilisation in a female fetus at risk of congenital adrenal hyperplasia.2 Arguments for screening prenatally are that early diagnosis of SCD has shown better outcomes.14-16,18 Additional large scale unbiased studies are needed to assess the screening performance of NIPT for detecting SCD.1,18,19 Apart from Turner syndrome, there is not yet reliable data on the clinical sensitivity of NIPT for SCD.18
For more information on sex chromosome differences, see the articles and links below:
Turner syndrome (45, X)
Huang AC, Olson SB, Maslen CL. A Review of Recent Developments in Turner Syndrome Research. J Cardiovasc Dev Dis. 2021 Oct 23;8(11):138. PMID: 34821691
Gravholt CH, Viuff MH, Brun S, et al. Turner syndrome: mechanisms and management. Nat Rev Endocrinol. 2019 Oct;15(10):601-614. PMID: 31213699
Shankar Kikkeri N, Nagalli S. Turner Syndrome. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554621/ [Accessed June 2024]
Sex chromosome trisomies (47, XXY; 47, XXX; 47, XYY)
Leggett V, Jacobs P, Nation K, Scerif G, Bishop DV. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review. Dev Med Child Neurol. 2010 Feb;52(2):119-29. PMID: 20059514
Tartaglia N, Howell S, Davis S, et al. Early neurodevelopmental and medical profile in children with sex chromosome trisomies: Background for the prospective eXtraordinarY babies study to identify early risk factors and targets for intervention. Am J Med Genet C Semin Med Genet. 2020 Jun;184(2):428-443. PMID: 32506668
Klinefelter syndrome (47, XXY)
Los E, Ford GA. Klinefelter Syndrome. [Updated 2021 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482314/ [Accessed June 2024]
Bonomi M, Rochira V, Pasquali D, et al. Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest. 2017;40(2):123-134 PMID: 27644703
Triple X syndrome (47, XXX)
Wigby K, D'Epagnier C, Howell S, et al. Expanding the phenotype of Triple X syndrome: A comparison of prenatal versus postnatal diagnosis. Am J Med Genet A. 2016 Nov;170(11):2870-2881 PMID: 27644018
Tartaglia NR, Howell S, Sutherland A, et al. A review of trisomy X (47,XXX). Orphanet J Rare Dis. 2010 May 11;5:8. PMID: 20459843
Otter M, Schrander-Stumpel CT, Curfs LM. Triple X syndrome: a review of the literature. Eur J Hum Genet. 2010 Mar;18(3):265-71. PMID: 19568271
Jacobs syndrome (47, XYY)
Sood B, Clemente Fuentes RW. Jacobs Syndrome. [Updated 2021 Oct 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557699/ [Accessed June 2024]
Bardsley MZ, Kowal K, Levy C, et al. 47,XYY syndrome: clinical phenotype and timing of ascertainment. J Pediatr. 2013 Oct;163(4):1085-94. PMID: 23810129
What about additional screening by NIPT (e.g. microdeletions and microduplications)?
Most NIPT companies offer options for additional screening of conditions other than the common three trisomies (21, 18 and 13). Examples are microdeletion and microduplication syndromes, rare trisomies (e.g. trisomy 15 or 16), as well as genome-wide structural anomalies (i.e. to identify unbalanced translocations where a parent may be a carrier of a balanced reciprocal translocation – see below).
Currently no national or international professional organizations support routine clinical implementation of additional screening by NIPT.2,18 The main argument against routine use is that reliable published data are not available. While positive predictive value may look encouraging in proof-of-concept studies, the data are overestimated for the general obstetric population. The clinical utility and sensitivity have not been demonstrated.18 The addition of these rare conditions to NIPT increases the false positive rate and decreases the positive predictive value of the test. This would result in more pregnant persons having diagnostic testing with an associated risk of miscarriage.
What about NIPT for twins or higher order multiples (e.g. triplets)?
Because the risk for pregnancy loss or complication associated with diagnostic testing may be higher in twin pregnancies20, a screening test more accurate than conventional screening is highly desirable. In 2017, the SOGC and CCMG recommended that NIPT in twins be undertaken with caution as additional validation studies and evaluation were needed before a recommendation could be made. Recommended screening modalities are (1) nuchal translucency (NT) with maternal age; (2) NT, maternal age and first trimester serum markers; (3) NT, maternal age plus first and second trimester serum markers, where available.2
In 2020, the International Society for Prenatal Diagnosis (ISPD) stated that there is now sufficient evidence to demonstrate high detection rate and positive predictive value and low false positive rate for twin pregnancies and that NIPT as a first trimester screening is appropriate.21 Consult your local genetics centre as funding may be available.
A 2021 review in Prenatal Diagnosis, states that NIPT in twin pregnancies can complement, but not replace, first trimester ultrasound evaluation.20 Overall, the observed performance of NIPT screening for trisomy 21 is comparable to singletons, and, while there are less data for trisomy 13 and 18, similar performance is expected.20 Ultrasound between 11-14 weeks can provide information about chorionicity, fetal anomalies (e.g. increased nuchal translucency, major structural anomalies), and abnormal maternal anatomy (e.g. Mullerian differences, adnexal masses).
Zygosity: In twin pregnancies, the combined fetal fraction is higher than in singleton pregnancies, but the fetal fraction per fetus is lower. For monozygotic twin pregnancies (identical, derived from the splitting of a single egg fertilized by one sperm) this is not a concern as both twins are expected to have the same genetic profile. For dizygotic twin pregnancies (non-identical, derived from fertilization of two eggs by two sperm), fetal fraction of each twin is important for test accuracy.20
Sex chromosome differences: Testing for sex chromosome differences is generally not available for non-singleton pregnancies.
Test failure/ No-Call result: For twin pregnancies, the median failure rate is reported to be 5.6% at the first blood draw, with a revised rate of 3.1% upon a second blood draw. The main reported reason is low fetal fraction, which is associated with high maternal BMI, in vitro fertilization, aneuploidy and other factors.21
High order multiples: Triplet and higher order pregnancies are rare. Observed detection rates for trisomy 21 and other common aneuploidies are not available. Test failure rate is also expected to be higher than for singleton and twin pregnancies.21
What about NIPT and in vitro fertilization?
Pregnancies conceived through in vitro fertilization (IVF) are increasing in number.22,23 Preimplantation genetic testing for aneuploidy (PGT-A) to screen embryos for chromosome differences prior to implantation is also being used more commonly.22,23 The resulting pregnancies have a lower rate of aneuploidy than those conceived without PGT-A, although the precise sensitivity and specificity are not known due to limited available data.22
Conventional first trimester screening methods are less accurate in IVF pregnancies as there are differences in analyte levels (e.g. lower PAPP-A) associated with an increased potential for false positive results.24 Additionally, these screening methods are not able to incorporate previous screening (e.g. by PGT-A which means a lower a priori risk than standard age-related risk) resulting in inaccurate assessments.22,23
NIPT for IVF pregnancies with or without PGT-A is the preferred method of prenatal screening. The sensitivity and specificity of NIPT has been validated for screening for common aneuploidies, more so than PGT-A.22,23
Diagnostic testing is not recommended unless NIPT screening result is high risk/positive, or ultrasound identifies a high risk.22,23
Clinicians are advised to offer NIPT with caution as the performance in IVF pregnancies may be altered.22-24 IVF pregnancies are reported to have lower fetal fraction223,24, a suspected reason for the higher test failure rate among these pregnancies.22-24 Successful results following a redraw and reanalysis are reported to be comparable to non-IVF pregnancies.24
For pregnancies where PGT-A was used and a euploid (typical chromosome number, not aneuploid) embryo was selected for implantation, the positive predictive value (PPV) of NIPT is lower and false positive rate is higher.23 This is expected as the PPV is based on the prevalence of a condition in a population, and the prevalence of chromosome differences in embryos that have undergone screening by PGT-A would be lower than the general population.
Regardless of conception method, all pregnant persons should be offered a first trimester ultrasound (at 11 to 14 weeks gestation) for accurate dating, determination of twin chorionicity, early anatomy assessment and nuchal translucency (NT) measurement. A second trimester ultrasound (18 to 22 weeks gestation) should be offered for evaluation of structural anomalies. Ultrasounds should be performed at centres with expertise in fetal ultrasound.2
Check online for updates as prenatal genomics is a rapidly evolving area. References and specifics about provincial availability can also be found online.
See Public Resources for the updated A guide to understanding prenatal screening tests to help expectant parents and their healthcare providers choose the right testing option, including NIPT.
Authors: S Morrison MS CGC, JE Allanson MD FRCPC and JC Carroll MD CCFP
GECKO on the run is for educational purposes only and should not be used as a substitute for clinical judgement. GECKO aims to aid the practicing clinician by providing informed opinions regarding genetic services that have been developed in a rigorous and evidence-based manner. Physicians must use their own clinical judgement in addition to published articles and the information presented herein. GECKO assumes no responsibility or liability resulting from the use of information contained herein.
References
1. Gil MM, Accurti V, Santacruz B, et al. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol 2017;50(3):302-314
2. Audibert F, De Bie I, Johnson JA, et al. 348-Joint SOGC-CCMG Guideline: Update on Prenatal Screening for Fetal Aneuploidy, Fetal Anomalies, and Adverse Pregnancy Outcomes. J Obstet Gynaecol Can 2017; 39(9):805-817
3. Wilson RD, SOGC genetics committee. Prenatal screening, diagnosis, and pregnancy management of fetal neural tube defects. J Obstet Gynaecol Can 2014; 36(10):927-939
4. Van den Hof MC, Wilson RD, et al. Fetal soft markers in obstetric ultrasound. J Obstet Gynaecol Can 2005;27(6):592-636
5. Bellai-Dussault K, Meng L, Huang T, Reszel J, Walker M, Lanes A, Okun N, Armour C, Dougan S. A 2-year review of publicly funded cell-free DNA screening in Ontario: utilization and adherence to funding criteria. Prenat Diagn. 2020 Jan;40(2):164-172
6. Carroll JC, Morrison S, Miller FA, et al. Anticipating the primary care role in genomic medicine: expectations of genetics health professionals. J Community Genet. 2021;12(4):559-56
7. Dougan SD, Okun N, Bellai-Dussault K, et al. Performance of a universal prenatal screening program incorporating cell-free fetal DNA analysis in Ontario, Canada. CMAJ 2021;193(30):E1156-E1163
8. Gekas J, Langlois S, Ravitsky V, et al. Non-invasive prenatal testing for fetal chromosome abnormalities: review of clinical and ethical issues. Appl Clin Genet 2016 4; 9:15-26
9. Gil MM, Quezada MS, Revello R, et al. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol 2015; 45(3):249-66
10. Cuckle H, Benn P, Pergament E. Cell-free DNA screening for fetal aneuploidy as a clinical service. Clin Biochem 2015; 48(15):932-41
11. Huang AC, Olson SB, Maslen CL. A Review of Recent Developments in Turner Syndrome Research. J Cardiovasc Dev Dis. 2021 Oct 23;8(11):138.
12. Gravholt CH, Viuff MH, Brun S, et al. Turner syndrome: mechanisms and management. Nat Rev Endocrinol. 2019 Oct;15(10):601-614.
13. Leggett V, Jacobs P, Nation K, et al. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review. Dev Med Child Neurol. 2010 Feb;52(2):119-29.
14. Tartaglia N, Howell S, Davis S, et al. Early neurodevelopmental and medical profile in children with sex chromosome trisomies: Background for the prospective eXtraordinarY babies study to identify early risk factors and targets for intervention. Am J Med Genet C Semin Med Genet. 2020 Jun;184(2):428-443.
15. Wigby K, D'Epagnier C, Howell S, et al. Expanding the phenotype of Triple X syndrome: A comparison of prenatal versus postnatal diagnosis. Am J Med Genet A. 2016 Nov;170(11):2870-2881.
16. Bardsley MZ, Kowal K, Levy C, et al. 47,XYY syndrome: clinical phenotype and timing of ascertainment. J Pediatr. 2013 Oct;163(4):1085-94
17. Bonomi M, Rochira V, Pasquali D, Balercia G, Jannini EA, Ferlin A; Klinefelter ItaliaN Group (KING). Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest. 2017 Feb;40(2):123-134
18. Christiaens L, Chitty LS, Langlois S. Current controversies in prenatal diagnosis: Expanded NIPT that includes conditions other than trisomies 13, 18, and 21 should be offered. Prenat Diagn. 2021 Sep;41(10):1316-1323
19. Soukkhaphone B, Lindsay C, Langlois S, et al. Non-invasive prenatal testing for the prenatal screening of sex chromosome aneuploidies: A systematic review and meta-analysis of diagnostic test accuracy studies. Mol Genet Genomic Med. 2021;9(5):e1654
20. Benn P, Rebarber A. Non-invasive prenatal testing in the management of twin pregnancies. Prenat Diagn. 2021;41(10):1233-1240
21. Palomaki GE, Chiu RWK, Pertile MD, et al. International Society for Prenatal Diagnosis Position Statement: cell free (cf)DNA screening for Down syndrome in multiple pregnancies. Prenat Diagn. 2021;41(10):1222-1232
22. Zwingerman R, Langlois S. Committee Opinion No. 406: Prenatal Testing After IVF With Preimplantation Genetic Testing for Aneuploidy. J Obstet Gynaecol Can. 2020 Nov;42(11):1437-1443
23. Klimczak AM, Seli E, Scott RT Jr. Noninvasive prenatal testing in women undergoing in vitro fertilization with preimplantation genetic testing. Curr Opin Obstet Gynecol. 2021 Jun 1;33(3):184-187
24. Society for Maternal-Fetal Medicine (SMFM), Ghidini A, Gandhi M, McCoy J, Kuller JA; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #60: Management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2021 Nov 2:S0002-9378(21)01187-X
Below are links to provincial prenatal screening program and information about whether or not NIPT can be obtained with provincial funding: [Reviewed May 2024]
|
Province |
Provincial screening |
NIPT funded |
|
Alberta |
MyHealth Alberta Prenatal Screening https://myhealth.alberta.ca/genetics/prenatal-screening-and-testing |
No |
|
British Columbia |
Perinatal Services BC Prenatal Screening http://www.perinatalservicesbc.ca/our-services/screening-programs/prenatal-genetic-screening |
Yes, in some circumstances |
|
Saskatchewan |
Information for public |
No |
|
Manitoba |
Prenatal Genetic Screening & Diagnosis Service - Winnipeg Regional Health Authority (wrha.mb.ca) https://wrha.mb.ca/genetics-and-metabolism/prenatal-genetic-screening/ |
Yes, following positive maternal serum screen |
|
Maritimes (New Brunswick [NB], Nova Scotia [NS], Prince Edward Island [PEI]) |
IWK Prenatal Screening |
Yes, in some circumstances NB, discuss with maternal fetal medicine specialist NS and PEI criteria found here: IWK Health Centre - Clinical Genomics - NIPT Information for Care Providers (nshealth.ca) https://iwkhealth.ca/clinical-genomics/testing-menu/nipt-information-care-providers |
|
Newfoundland and Labrador |
contact local genetics centre |
Yes, criteria found here |
|
Ontario |
Prenatal Screening Ontario |
Yes, criteria found here |
|
Quebec |
Québec prenatal screening program |
Genetic services and test access and criteria for the Territories are generally administered through provincial programs
- Alberta and Northwest Territories and Nunavut (Kitimeot)
- British Colombia and Yukon
- Manitoba and Nunavut (Kiviliq)
- Ontario and Nunavut (Baffin)