Download the PDF here. Download the point of care tool to assist identifying patients most likely to benefit from referral to genetics or the more comprehensive review, the GECKO deep dive containing more on risks, benefits, limitations, screening and management, as well as for the made for practice.
Bottom line:
Hereditary breast/ovarian cancer predisposition refers to an inherited increased lifetime risk to develop breast and/or ovarian cancer and accounts for about 5-15% of these cancers. The actual cancer risk depends upon the gene involved as well as personal and family history factors. Primary care practitioners (PCPs) have a role in identifying those who are at increased risk to carry a pathogenic/likely pathogenic variant (P/LP) in a gene associated with increased cancer risk, as well as facilitating familial testing when there is a known genetic P/LP. Genetic testing may be considered where there is a personal and/or family history of:
- multiple (>3) cancer diagnoses of the same or related type on the same side of the family.
- Cancers related to hereditary breast/ovarian cancer predisposition genes are breast, ovary, prostate, and pancreas.
- young age at diagnosis (<50y).
- rare cancer diagnosis (e.g. male breast cancer).
- at-risk ancestry (e.g. Ashkenazi Jewish).
PCPs also have a role in coordinating high-risk screening (e.g. MRI) and referring for specialist consultation for consideration of risk-reducing management. Genomic knowledge is rapidly expanding. Thus, staying up-to-date with new referral criteria and screening recommendations, and possibly re-referring patients for genetic consultation important roles for PCPs.
Reviewed July 2025
What is hereditary breast and ovarian cancer?
All cancer is genetic, but not all cancer is inherited. This means that all cancer is the product of pathogenic/likely pathogenic (P/LP) variants (what used to be called mutations) in one or more genes that result in uncontrollable cell growth. However, most of these genetic changes are acquired and not inherited from a parent. In fact, only about 5-10% of breast cancer and about 15% of ovarian cancer is thought to be the result of an inherited P/LP variant in a cancer predisposition gene.1 Often cancer predisposition genes have roles in regulating cell growth (tumor suppressor genes) or repairing damaged DNA.
Individuals with an inherited predisposition to cancer have a significantly increased risk for breast (2-6x) and/or ovarian (10-30x) cancer. The actual risk depends on which gene has a P/LP variant as well as personal and family history factors. Not every individual with an inherited predisposition will develop cancer (reduced penetrance) and not every individual in a single family will develop the same cancer or have the same age of onset (variable expressivity).
Who to consider referring for a genomic assessment for hereditary breast/ovarian cancer?
Genomic centres across Canada have their own criteria for referral and genetic testing. Check out the GECKO site for how to contact your local genomics centre and for links to provincial breast cancer screening programs.
A first step to assessing those who would benefit from a genetic assessment is eliciting a family history. Validated tools exist to facilitate identifying those who are at increased risk to have a hereditary predisposition to breast/ovarian cancer and there are general red flags to identify those at risk of any hereditary cancer predisposition (GECKO point of care tool: General hereditary cancer red flags). More specific red flags to explore when there is a suspicion for hereditary breast/ovarian cancer can be found in our GECKO point of care tool: hereditary breast/ovarian cancer red flags. Reach out to your local genetics provider by eConsult or phone if you have a question about referral, family history, screening or further assessment.
General personal/family history cancer predisposition red flags:
In general, red flags that raise the suspicion of a hereditary cancer predisposition are:
- Multiple relatives (<3) with the same or related cancer diagnoses, on the same side of the family
- Cancers related to hereditary breast/ovarian cancer predisposition genes are breast, ovary, prostate, and pancreas
- Young age at diagnosis (e.g. breast cancer <50y, pancreatic cancer <45y)
- Several affected generations
- Multiple primaries in one individual (e.g. bilateral breast cancer, breast and ovarian cancer)
- High risk cancer diagnosis (e.g. triple negative breast cancer diagnosed <60y, high-grade metastatic castration resistant prostate cancer)
- Rare/unusual cancer diagnoses (e.g. male breast cancer)
- Ancestry where there is a higher carrier frequency (e.g. The general population carrier frequency for pathogenic variants in BRCA1 and BRCA2 is 1 in 400 whereas in those of Ashkenazi Jewish ancestry the carrier frequency is 1 in 40)
Important pieces of information to clarify are: (1) cancer diagnosis; (2) age at diagnosis; (3) relation to patient; (4) ancestry.
The CanRisk Web Tool and the IBIS Breast Cancer Risk Evaluation Tool are validated models used by many genomic centres. These incorporate personal health history (e.g. breast density, age at menses) as well as family history to estimate life time cancer risk as well as the likelihood an individual has an inherited cancer risk.
With any suspected genetic condition, the best person in whom to begin genetic testing is always the person most likely to carry a pathogenic variant in a candidate gene. With hereditary cancer predispositions, this person is typically the one diagnosed with cancer at the youngest age (e.g. breast cancer at age 40), the one diagnosed with multiple cancers (e.g. breast and ovarian cancer, bilateral breast cancer), or the one diagnosed with a rare cancer (e.g. male breast cancer). While unaffected individuals are not typically first in a family to be offered genetic testing, if the probability is high enough (>5-10%) using an established risk model, testing may be considered when another more appropriate relative is not available.
What do the genetic test results mean?
For more information on genomic testing and results see our point of care tool. Most provincial genomic laboratories will have board-certified genetic counsellors who can answer your questions about genetic test results and are happy to take your calls.
Positive result: A P/LP variant in a cancer predisposition gene was identified. If the gene is associated with the person’s cancer diagnosis, this explains their cancer. Results may be used by oncology to inform treatment and/or surveillance plan and may allow for access to new effective therapies such as PARP inhibitors. The individual is likely at higher risk for subsequent cancer diagnoses. Additional screening and surveillance for other cancers may be indicated. First degree relatives (parents, siblings, offspring) have a 50% chance to inherit this gene change and to also be at risk. Relatives can have genetic testing for this familial variant.
Negative/uninformative result: No variants of clinical significance were identified in any of the genes on the cancer panel. Given the comprehensiveness of cancer gene panels, a negative result significantly reduces the likelihood of a hereditary cancer condition in the person tested. Depending on the family history, other relatives may still be eligible for genetic testing. Screening and surveillance recommendations for the individual and their relatives will be based on personal and family history, which may include high risk breast screening with MRI.
True negative: The familial variant was not detected. This is where a pathogenic/ likely pathogenic variant in a cancer predisposition gene has already been identified in a relative. Testing was solely to detect the presence or absence of the familial pathogenic variant. This individual is not at increased risk for the familial cancer. Depending on the family history, population-based cancer screening is still recommended.
Variant of uncertain significance (VUS): A variant in a gene where the significance is not yet known. The laboratory cannot confidently determine if the gene variant identified is pathogenic or benign as available evidence is insufficient or conflicting. This result is reassuring as pathogenic variants in high/moderate risk cancer genes have been ruled out, however a hereditary cancer condition cannot be completely excluded. A board-certified genetic counsellor or geneticist can help to interpret the laboratory report. No changes to medical management are indicated. Family members are typically not offered genetic testing for a VUS. Clinics and laboratories differ in their re-contact protocols, but generally a patient would be encouraged to re-contact their genetics provider in 2-5 years for updates on re-classification of the VUS they carry. Re-classification of a VUS could mean the variant is now confidently determined to be pathogenic or benign.
Cascade genetic testing: Once a P/LP variant has been identified in a family, other relatives are eligible to have genetic testing for that variant and can have more accurate risk assessments, screening and management recommendations. Genetic counsellors can help facilitate communication of results among relatives by providing/sending family letters with de-identified information after ensuring appropriate consent to share genetic information is documented. PCPs can play a key role in cascade testing of hereditary conditions by discussing the benefits of sharing results, of genetic testing and by making referrals.
What are the benefits and considerations of genetic testing?
Benefits:
- A positive result can explain why a person developed cancer and confirm a hereditary predisposition in the family
- Oncology may use the result to inform treatment
- Additional screening, surveillance and/or surgical recommendations may be suggested
- Clinical intervention for those with known hereditary cancer predispositions can improve outcomes
- At-risk relatives can be identified and other relatives without the familial variant can be reassured. Both can be given more accurate risk assessments
- Positive health behaviours can be reinforced
Considerations:
- Some may experience psychological distress over the:
- increased risk to develop cancer
- possibility of passing an increased cancer risk to offspring
- uncertainty of a negative result or a variant of uncertain significance
- uncertainty of a cancer diagnosis, as not everyone with a genetic predisposition will develop cancer
- Family issues such as confidentiality concerns or estrangement may inhibit the transfer of information between relatives
- In Canada, the Genetic Non-Discrimination (GNA) Act became law in 2017. This law protects Canadians and their genomic information. Some key points of the law are that GNA prohibits:
- Discrimination based on genomic characteristics
- Providers of goods and services (including insurance) from:
- requesting or requiring a genomic test
- requesting or requiring the disclosure of genomic test results either past or future
- Federally regulated employers from using genomic test results in decisions about hiring, firing, job assignments, or promotions
What are the cancer risks associated with an inherited predisposition?
Many of the genes associated with an inherited predisposition to breast/ovarian cancer have a role in the cell’s ability to repair double-stranded DNA breaks (homologous repair), however associated cancers and lifetime risks vary between genes. Cancer gene risks can be categorized as moderate (~2-3x) to high (4x or higher) lifetime risk. Figure 1 shows gene-associated breast cancer risks. Additionally, an individual's personal risk may differ from published lifetime risks, depending on age, medical history and family history.
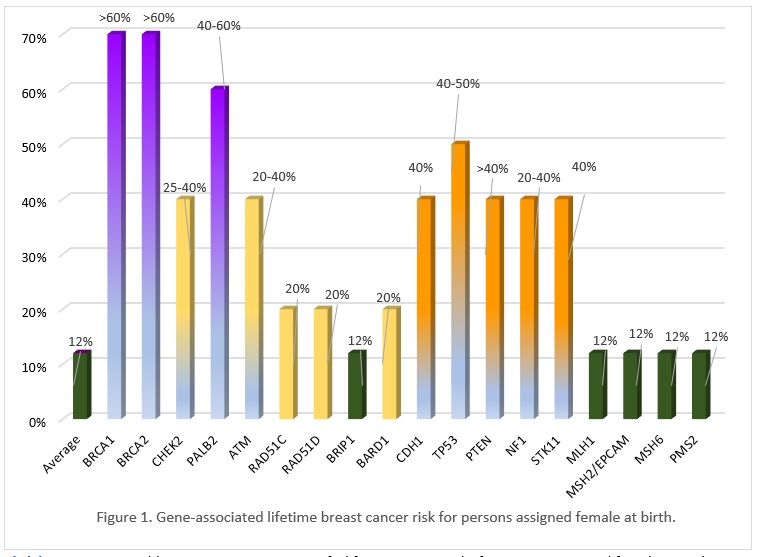
Click here to view a table summarizing gene specific lifetime cancer risks for persons assigned female or male at birth for breast, ovary, prostate and pancreas cancer, as well as other possible associated cancers.
Gene specific cancer surveillance and management recommendations
A multidisciplinary approach involving medical, surgical, and genetic specialties is the best approach to managing those with a hereditary cancer predisposition. If your patient has not yet had genetic counselling to discuss their hereditary condition, consider referring so that appropriate specialists, perhaps those with special experience with hereditary cancer, can be identified and included in the care of your patient. If your patient had genetic counselling several years ago, consider reaching out to your local genetics specialist by phone or eConsult to inquire about any updated screening recommendations, as they evolve over time. More details about screening and surveillance recommendations per gene can be found in the GECKO Deep Dive.
Genetic testing of children and minors
Guidelines recommend that unless there is evidence for timely medical benefit or actionable management in the pediatric period, genetic testing for adult-onset conditions should be deferred until the child/minor is capable of deciding whether to be tested. Clinicians are not obligated to comply with requests from parents to test healthy children. There may be exceptions such as where the wait for testing itself may cause an undue psychological burden for the child. Requests for testing by competent, well-informed adolescents may be considered in conjunction with comprehensive genetic counselling.
See the GECKO Deep Dive for the full reference list.
Related point of care tools and key links:
Authors: S Morrison MS CGC, JE Allanson MD FRCPC, S Walji MD CCFP and JC Carroll MD CCFP
Disclaimer:
· GECKO is an independent not-for-profit program that does not accept support from commercial or non-academic entities.
· GECKO aims to aid the practicing non-genetics clinician by providing informed resources regarding genetic/genomic conditions, services and technologies that have been developed in a rigorous and evidence-based manner with periodic updating. The content on the GECKO site is for educational purposes only. No resource should be used as a substitute for clinical judgement. GECKO assumes no responsibility or liability resulting from the use of information contained herein.
· All clinicians using this site are encouraged to consult local genetics clinics, medical geneticists, or specialists for clarification of questions that arise relating to specific patient problems.
· All patients should seek the advice of their own physician or other qualified clinician regarding any medical questions or conditions.
· External links are selected and reviewed at the time a page is published. However, GECKO is not responsible for the content of external websites. The inclusion of a link to an external website from GECKO should not be understood to be an endorsement of that website or the site’s owners (or their products/services).
· We strive to provide accurate, timely, unbiased, and up-to-date information on this site, and make every attempt to ensure the integrity of the site. However, it is possible that the information contained here may contain inaccuracies or errors for which neither GECKO nor its funding agencies assume responsibility.



