
Download the GECKO Messenger PDF, the quick reference GECKO on the run, and/or the point of care tool.
Last updated October 2014
Bottom line: Factor V Leiden (FVL) is the most common genetic risk factor for venous thromboembolism (VTE) and occurs in about 5% of the Caucasian population. It is considered to be a moderate risk factor for VTE, and its clinical expression is influenced by co-existing genetic factors, in addition to acquired and circumstantial risk factors. FVL status seems to have little or no effect on recurrence risk of VTE, does not influence treatment following a VTE event, and is not an indication for primary prophylactic treatment in asymptomatic carriers. There is consensus that in some circumstances genetic testing for FVL has utility, as carriers should be educated about circumstances that might increase the likelihood of VTE, signs and symptoms of VTE, and the potential need for prophylactic anticoagulation in high-risk circumstances.
What is factor V Leiden?
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a multifactorial disease resulting from a combination of genetic, environmental and circumstantial risks1. DVT is estimated to occur in about 1 per 1000 persons per yearand is predominant in older age groups 1,2. PE occurs less often. Individuals who have experienced a VTE are at increased risk for a recurrent episode1,3.
Factor V is a clotting factor with activity regulated by the anti-coagulant activated protein C (APC). A single base substitution in the factor V gene (G>A at position 1691) results in the elimination of one of APC’s three cleavage sites. This mutation causes factor V to be inactivated more slowly by APC, generating more thrombin and consequently increasing the potential for clot formation. This specific mutation in the factor V gene is called factor V Leiden (FVL).3
FVL is the most common genetic risk factor for VTE1,3. Other co-existing inherited and acquired disorders and circumstantial risk factors affect clinical expression of FVL, for example, also carrying the prothrombin variant 20210G>A (carrying both the FVL and 2010G>A alleles occurs in about 1/1000 of the general population), obesity, age, immobility due to injury, surgery or air travel, oral contraceptives, hormone replacement therapy (HRT), selective estrogen receptor modulator (SERMs) or pregnancy. Heart attack, arterial thrombosis and ischemic stroke are not associated with FVL.1
This GEC-KO Messsenger will focus exclusively on FVL. FVL is inherited in an autosomal dominant manner. About 5% of the Caucasian population carries the FVL mutation with the prevalence being lower in other ethnicities (about 2% in Hispanic Americans and about 1% in African Americans)1,2,3. Homozygosity for FVL (both copies of the FV gene having the mutation) occurs much less often, in about 1 per 5000 Caucasian individuals1,3. About 20-25% of individuals with VTE, 40-50% of individuals with recurrent VTE or estrogen related thrombosis, and about 50% of individuals with familial thrombophilia are found to have FVL1.
Red flags to consider genetic testing or genetic consultation?
Genetic testing for FVL may have clinical utility in certain circumstances (table 1), however there is no evidence that knowledge of FVL carrier status influences treatment following a VTE event to avoid recurrence. Similarly, there is no evidence that primary prophylactic treatment in asymptomatic carriers improves clinical outcome.1,3
Table 1. Summary of scenarios where genetic testing for FVL may be indicated.1,4
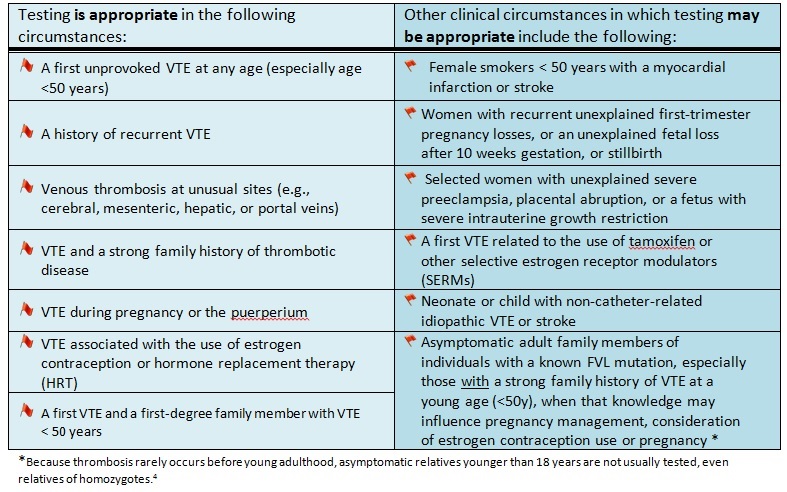
FVL testing is not routinely recommended:1,4
- For the general population
- During routine pregnancy screening
- Before prescribing estrogen contraception, HRT or SERMs
- For prenatal testing or screening of asymptomatic newborns, neonates, and children
- For patients with a personal or family history of arterial thrombosis (acute coronary syndrome or stroke), unless unexplained in an individual under age 50
What does the genetic test result mean?
Population studies suggest that about 10% of FVL carriers develop VTE over their lifetime; the risk is higher for those with a family history of thrombophilia (~25-40%)1. Carriers of FVL and individuals who are homozygous (carrying the FVL change in both FV genes) have an increased lifetime risk for VTE, but that absolute risk is very small. For example, in the general population women under age 40 have a background risk of VTE of 8/100,000, whereas female FVL carriers of the same age have a VTE risk of 24/100,000 and the homozygote risk for women in that age range is 8/10,000.1,2 FVL heterozygosity has, at most, a moderate effect on recurrence risk. FVL homozygosity effect on recurrence risk is not well defined. FVL carriers do not, however, have an increased risk for mortality or morbidity.1
Surveillance and Management
Individuals at high risk for clotting
Those with a history of recurrent VTE, especially at a young age, or those with a strong family history of VTE at a young age should, in addition to FVL testing, be screened for:1
- Protein C activity
- Antithrombin activity
- Free Protein S antigen or Protein S activity
- Prothrombin variant 20210G>A
Asymptomatic FVL carriers
Table 2. Management recommendations for asymptomatic FVL carriers.
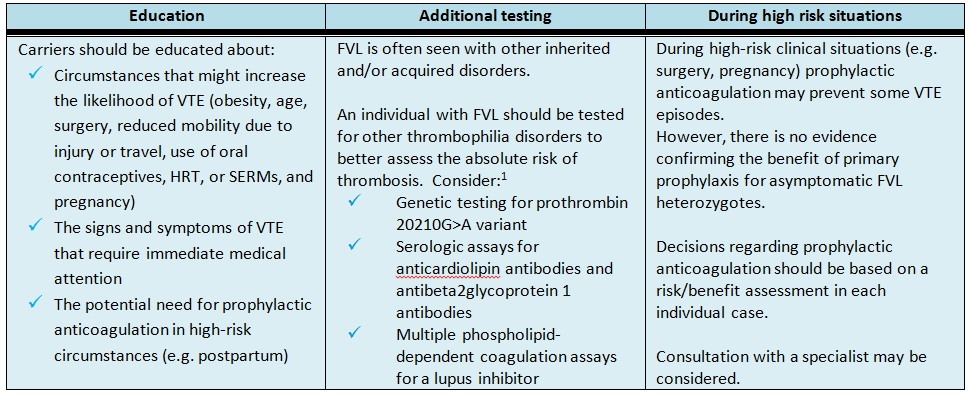
Following a VTE event
Management of individuals with FVL depends on clinical circumstances. The first acute VTE should be treated according to standard guidelines7. In the absence of other indications, FVL carrier status is not an indication for prolonged treatment.1
In cases of unprovoked VTE in a FVL carrier, for FVL homozygotes, and for those with multiple thrombophilic disorders (e.g. carriers of both FVL and the prothrombin 20210G>A variant), long-term antithrombotic prophylaxis should be considered; however, the true risk-benefit ratio of extended anticoagulation treatment is not known.1,4
FVL and pregnancy
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recently published guidelines regarding diagnosis, treatment and thromboprophylaxis of VTE in pregnancy and postpartum which are available on the website www.sogc.org under Clinical Practice Guidelines.6
Resources for health professionals
[1] Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13(1): 1-13
[2] Martinelli I, De Stefano V, Mannucci PM. Inherited risk factors for venous thromboembolism. Nat Rev Cardiol 2014; 11(3):140-56
[3] Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med 2011;13(1):67-76
[4] Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA, ACMG Factor V. Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3(2):139-48
[5] Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(6 Suppl):381S-453S.
[6] Chan WS, Rey E, Kent NE; VTE in Pregnancy Guideline Working Group, Chan WS, Kent NE, Rey E, Corbett T, David M, Douglas MJ, Gibson PS, Magee L, Rodger M, Smith RE. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can 2014; 36(6):527-53
[7] Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:454S–545S
Resources for patients and the public
Thrombosis Canada http://thrombosiscanada.ca/
Authors: S Morrison MS CGC, JC Carroll MD CCFP and JE Allanson MD FRCPC
Disclaimer:
· GECKO is an independent not-for-profit program that does not accept support from commercial or non-academic entities.
· GECKO aims to aid the practicing non-genetics clinician by providing informed resources regarding genetic/genomic conditions, services and technologies that have been developed in a rigorous and evidence-based manner with periodic updating. The content on the GECKO site is for educational purposes only. No resource should be used as a substitute for clinical judgement. GECKO assumes no responsibility or liability resulting from the use of information contained herein.
· All clinicians using this site are encouraged to consult local genetics clinics, medical geneticists, or specialists for clarification of questions that arise relating to specific patient problems.
· All patients should seek the advice of their own physician or other qualified clinician regarding any medical questions or conditions.
· External links are selected and reviewed at the time a page is published. However, GECKO is not responsible for the content of external websites. The inclusion of a link to an external website from GECKO should not be understood to be an endorsement of that website or the site’s owners (or their products/services).
· We strive to provide accurate, timely, unbiased, and up-to-date information on this site, and make every attempt to ensure the integrity of the site. However, it is possible that the information contained here may contain inaccuracies or errors for which neither GECKO nor its funding agencies assume responsibility.


