
Download the comprehensive GECKO Messenger PDF. For a more concise summary view the GECKO on the run. View the point of care tool containing a road map of possible genetic tests and consultations for the individual with autism spectrum disorder. Access an education module with case-based learning here.
Last reviewed September 2022
Bottom line: Autism spectrum disorder (ASD) is a complex, genetically influenced disorder, affecting about 1 in 68 children. ASD is highly variable both in presentation and in etiology. In families where one child has ASD, the risk to subsequent siblings is about 10-19%. About 15-40% of individuals with ASD will have an identifiable contributing genetic cause. First tier genetic investigations for ASD are chromosomal microarray and fragile X syndrome testing. If an individual is determined to have ASD plus (signifying additional co-morbidities such as congenital anomalies, dysmorphic features, neurological symptoms (e.g. seizures)) additional investigations may be considered. A genetic diagnosis can potentially lead to guideline-based surveillance and management, tailored treatment options, opportunities to participate in clinical trials, information regarding natural history and prognosis, familial testing and more accurate recurrence risk counselling. Primary care providers who feel confident providing pre- and post –test counselling may be able to arrange first tier genetic investigations prior to or concurrent with referral for genetic consultation. Local laboratories may limit which providers can order these tests.
What is autism spectrum disorder?
Autism spectrum disorder (ASD) is a complex heterogeneous group of neurodevelopmental disorders affecting brain function and behaviour. The core features of ASD include pervasive impairments in communication and social interaction, repetitive behaviours and/or restricted interests1.
How common is ASD?
Recent surveillance data suggest that about 1 in 68 children is affected by ASD with males diagnosed about four times more often than females1,2.
How is ASD diagnosed?
Diagnosis of ASD is based on clinical criteria, the most recent of which are found in the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition [DSM-5]. While the average age at diagnosis is 4 years, a reliable diagnosis can be made by age 2 as most children with ASD will show signs if carefully screened. A child may first be brought to attention following routine screening using a tool such as the validated Modified Checklist for Autism in Toddlers-Revised (M-CHAT-R™). An ASD diagnosis can be made by a qualified professional using the DSM-5 criteria. For more on ASD diagnosis in the primary care setting, please see the excellent review by Anagnoustou and colleagues.1
What causes autism spectrum disorder?
ASD is a genetically influenced disorder caused by genetic, epigenetic (factors that affect gene expression and activity) and possibly non-genetic (environmental) factors1,3,4. For couples who have a child with ASD, the chance for each of their subsequent children to be diagnosed with ASD is about 10-19%3,5,6. Recurrence risks can sometimes be more accurately predicted if a genetic etiology is known.
ASD is extremely heterogeneous and several hundred genes have been implicated in ASD susceptibility. This likely explains the variability in phenotype amongst individuals with ASD, even within the same family1,3,4. About 15-40% of individuals with ASD will have an identifiable contributing genetic cause, depending on the study population and the type of genetic technology used3,7. Genetic changes leading to predisposition to ASD can be inherited from a parent or can be de novo (new in the affected individual). One study found that in greater than half the families (~69%) where more than one child had ASD, each affected sibling had a different ASD-relevant gene change8. These statistics speak to the complexity of ASD etiology.
About 25% of individuals with ASD will have complex ASD or ASD plus, meaning that autistic features are accompanied by congenital anomalies, dysmorphic features and/or neurological findings (see Figure 1 in What are the red flags that suggest genetic consultation and/or genetic testing?).
What are the red flags that suggest genetic consultation and/or genetic testing?
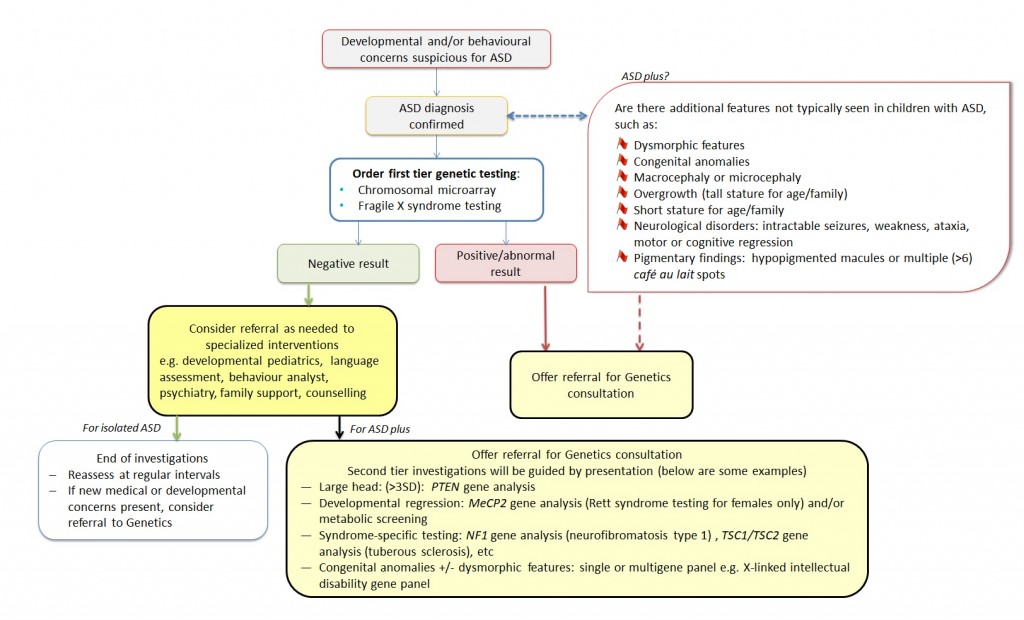
Figure 1. A road map of possible genetic tests and consultations for the individual with ASD. NOTE: Local laboratories may limit which providers can order first tier investigations. Contact your local genetics centre for more.
Once an ASD diagnosis is confirmed, a provider can consider the red flags above to determine if the individual has isolated ASD or ASD plus. A genetics referral could be made at that time. Alternatively, a provider who is comfortable providing pre-test counselling and post-test follow-up could begin the first tier genetic testing and refer upon receipt of results. Local laboratories may limit which providers can order these tests. Contact your local genetics centre for more.
What can my patient expect at a genetic consultation for autism spectrum disorder?
The role of a clinical geneticist is to identify the etiology of the ASD where possible, with the hope of improving management of the individual and providing genetic counselling for the family9. A clinical genetics consultation includes a review of the patient’s prenatal, perinatal, medical and family histories, and a physical examination to document growth parameters (such as head circumference) and look for evidence of dysmorphic features1.
In cases of isolated or apparently non-syndromic ASD, recommendations have been published outlining appropriate genetic investigations3,10. In some settings, these investigations may be arranged without a genetics assessment. Ordering providers should be confident in providing pre- and post-test counselling, test interpretation and appropriate follow up. As some local laboratories may limit which providers can order these tests, contact your local genetics centre prior to your first time ordering.
The Canadian College of Medical Geneticists (CCMG) recommends that chromosomal microarray and fragile X syndrome testing be the first tier laboratory investigation for any male or female whose ASD and/or other developmental disability is unexplained after a thorough history and physical examination10. Second tier investigations may be guided by presentation, with genetic testing for MeCP2 for suspected Rett syndrome in females with language and motor regression, PTEN analysis for those with extreme macrocephaly and a family history of cancers suggestive of Cowden syndrome, or other genetic testing for specific syndromes if the history and physical examination are suggestive (for example, neurofibromatosis type 1 or tuberous sclerosis). Clinical geneticists may offer a next-generation sequencing panel which could include tens to thousands of genes, or in some cases, whole exome sequencing (WES). The exome makes up about 2% of all the genetic information and is the coding region (exons) of the genetic sequence. WES reads through each base of the exome and compares that of an affected individual to reference databases looking for meaningful differences.
Screening for inborn errors of metabolism is not routinely recommended unless there are features on history or physical exam which create suspicion (such as motor or cognitive regression, seizures, unexplained periodic encephalopathy, etc)3. A Canadian diagnostic protocol (Treatable Intellectual Disability Endeavor, TIDE) has been published for the purpose of metabolic screening in cases of intellectual disability and developmental delay and can be found online at http://www.treatable-id.org/.
What are the benefits of genetic consultation, with or without genetic testing?
Advances in genomic technology have provided the opportunity for many families to receive an answer to the question ‘why does my child have ASD’. While, historically, individuals with ASD would often be grouped together, a genetic diagnosis may assist individuals, health care providers and families to tailor and apply personalized genomic medicine approaches including but not limited to:
- Personalized care: If a genetic diagnosis is found to explain the ASD, information may be available regarding prognosis and medical management7,9
- Management: For example, in the case of PTEN gene mutations, in addition to ASD, affected individuals and possibly their family members may be at higher risk for benign and malignant tumours of the thyroid, breast, and endometrium as well as hamartomatous intestinal polyps. Specific surveillance guidelines can be applied.
- Prognosis: A genetic diagnosis can aid families and health care providers in preparing for an individual’s future care and needs.
- More accurate recurrence risk (RR) counselling for families:
- In the absence of an identifiable genetic etiology, a RR range can be quoted for families based on empirical studies. For families where one child has ASD, the RR is about 10-19%5,6. Males are at higher risk than females. Couples with two children with ASD have a higher RR, around 30%3. The more distant an affected relative is, the lower the RR. The risk for half-siblings is about 3%6.
- If a genetic etiology is identified, the RR depends on how the responsible genetic change is inherited.
- Autosomal recessive disorders: 25% RR, e.g. Smith-Lemli-Optiz syndrome
- Autosomal dominant disorders: 50% RR, g. Cowden syndrome (PTEN) or neurofibromatosis (NF1) if one parent is also affected.
- X-linked disorders: 50% RR, when the mother is a carrier of the changed gene, however clinical presentation in females may be different from that in males e.g. fragile X syndrome
- de novo disorders (a new gene change in the affected individual, not inherited from either parent) <1% RR for siblings
- Support:
- While most genetic conditions are relatively rare, families around the world with a rare condition may be connected via the internet. Many genetic conditions have well-organized virtual networks where individuals and their families can share experiences and get advice. Many families obtain information about the latest research developments from such networks.
- Even in the absence of a genetic diagnosis families can benefit from genetic counselling. A genetic counselling session can provide individuals and families with information, psychosocial support both immediate and long term, guidance and help to prepare for the future, facilitate family communication, and aid in decision making11.
What are the limitations and complexities of genetic testing for autism spectrum disorder?
- Lack of a specific genetic etiology: Not all individuals will have an identifiable genetic diagnosis
- While the genetic contribution to ASD has been well established, today’s technology and knowledge will identify a genetic etiology in about 15-40% of individuals with ASD. The diagnostic yield in those with ASD plus is at the higher end of the range3,7,9,12.
- Individuals and families who attend a genetic consultation with the expectation of receiving a diagnosis may feel disappointed. Over time, as genomic information is better understood and as ASD research initiatives continue, re-referral to Genetics can be considered.
- Uncertain results: As genetic testing technologies advance, massive amounts of information are generated. Not every genomic variation has been classified as either benign or pathogenic. These variants are referred to as variants of uncertain significance (VUS). This sort of result can neither confirm nor rule out a specific genetic etiology. Variants are periodically re-classified. If an individual is found to have a VUS, re-contacting the local genetics clinic for updates could be considered.
- ASD ‘risk variant’ with incomplete penetrance: This type of genetic change may be found in an individual who does not have ASD, suggesting it is not the sole cause of an individual’s ASD, and additional genetic or non-genetic factors are necessary for ASD to develop. In this circumstance it is difficult to determine the chance of familial recurrence. This genetic change may have been inherited from an affected, mildly affected or even unaffected parent. This is one of the most difficult scenarios for genetic counseling3.
Where do I refer my patient?
Find the contact information for your local genetics centre here. Do not hesitate to call and ask to speak to a genetic counsellor or geneticist prior to referral if you have questions. S/he can also help you determine the ordering requirements of your local laboratory (there are typically specific requisitions for genetic testing, and some labs have restrictions on who can order these tests). For the most meaningful appointment for your patient and appropriate triaging, it is important to include any previous investigations and consultation notes (e.g. genetic test results, psychological assessments, brain imaging) along with your referral.
For community support and research opportunities for your patient and his/her family, see Autism Speaks Canada.
Screening and Management
For guidance on managing and supporting individuals with essential or non-syndromic ASD, see the review by Anagnoustou and colleagues1 which outlines treatments, interventions and their effectiveness.
Surveillance and management of individuals with complex or ASD plus will vary depending on the individual’s clinical presentation and what, if any, genetic diagnosis is made. Connect with your local genetics specialist for assistance.
Resources for more information
Disorder resources:
- Autism Speaks Canada: autismspeaks.ca
- Fragile X Research Foundation of Canada: fragilexcanada.ca
- Ontario Rett Syndrome Association (MeCP2): rett.ca
- PTEN Hamartoma Tumor Syndrome Foundation Cowden syndrome: http://www.ptenfoundation.org
- Unique: Information and support for families affected by rare chromosomal disorders and their providers: rarechromo.org
- Orphanet: Information and resources on rare diseases (and orphan drugs): orpha.net
Genetic and genomic resources, including more on whole exome sequencing (WES):
- Centre for Genetic Medicine (Hospital for Sick Children) > Genetic Education
- Undiagnosed Disease Network (UDN) > Genetic Resources > An introduction to genetic testing
- Clinical Sequencing Exploratory Research (CSER) Toolkit designed to help non-geneticist healthcare providers to understand genomic medicine and genome sequencing
- Epigenetics: Genetics Science Learning Center: genetics.utah.edu/content/epigenetics
Autism Research
- MSSNG Project https://www.mss.ng/
References
- Anagnostou E, Zwaigenbaum L, Szatmari P, et al. Autism spectrum disorder: advances in evidence-based practice. CMAJ 2014;186(7):509-19
- Szego MJ, Zawati MH. Whole Genome Sequencing as a Genetic Test for Autism Spectrum Disorder: From Bench to Bedside and then Back Again. J Can Acad Child Adolesc Psychiatry 2016;25(2):116-21
- Carter MT, Scherer SW. Autism spectrum disorder in the genetics clinic: a review. Clin Genet 2013;83(5):399-407
- Masi A, DeMayo MM, Glozier N, et al. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci Bull 2017;33(2):183-193
- Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics 2011;128:e488-95.
- Sandin S, Lichtenstein P, Kuja-Halkola R, et al. The familial risk of autism. JAMA 2014;311(17):1770-7
- Yuen RKC, Merico D, Bookman M, et al. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat Neurosci 2017;20(4):602-61
- Yuen RKC, Thiruvahindrapuram B, Merico D, et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat Med 2015;21(2):185-91
- Schaefer GB, Mendelsohn NJ; Professional Practice and Guidelines Committee. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med 2013;15(5):399-407
- Duncan AM, Chodirker B. Use of array genomic hybridization technology for constitutional genetic diagnosis in Canada. Paediatr Child Health 2011;16(4):211-2.
- Bernhardt BA, Biesecker BB, Mastromarino CL. Goals, benefits, and outcomes of genetic counseling: client and genetic counselor assessment. Am J Med Genet 2008;94(3):189-97.
- Miles JH. Complex Autism Spectrum Disorders and Cutting-Edge Molecular Diagnostic Tests 2015;314(9):879-80
Authors: S Morrison MS CGC, JC Carroll MD CCFP, MT Carter MD FRCPC FCCMG, and JE Allanson MD FRCPC FCCMG
Disclaimer:
· GECKO is an independent not-for-profit program that does not accept support from commercial or non-academic entities.
· GECKO aims to aid the practicing non-genetics clinician by providing informed resources regarding genetic/genomic conditions, services and technologies that have been developed in a rigorous and evidence-based manner with periodic updating. The content on the GECKO site is for educational purposes only. No resource should be used as a substitute for clinical judgement. GECKO assumes no responsibility or liability resulting from the use of information contained herein.
· All clinicians using this site are encouraged to consult local genetics clinics, medical geneticists, or specialists for clarification of questions that arise relating to specific patient problems.
· All patients should seek the advice of their own physician or other qualified clinician regarding any medical questions or conditions.
· External links are selected and reviewed at the time a page is published. However, GECKO is not responsible for the content of external websites. The inclusion of a link to an external website from GECKO should not be understood to be an endorsement of that website or the site’s owners (or their products/services).
· We strive to provide accurate, timely, unbiased, and up-to-date information on this site, and make every attempt to ensure the integrity of the site. However, it is possible that the information contained here may contain inaccuracies or errors for which neither GECKO nor its funding agencies assume responsibility.


